In modern animal production, maintaining intestinal health is an everyday challenge. Bacteria such as E. coli and Salmonella are not just health risks – they undermine performance, feed efficiency, and animal welfare. Their ability to attach to the gut epithelium initiates inflammation and stress, setting off a cascade that affects productivity and ultimately profitability.
To counter this, yeast cell wall products have become an established part of nutritional strategies. Their mannan oligosaccharides (MOS) can bind undesirable bacteria, limiting their adhesion to the intestinal mucosa, and together with β-glucans support immune modulation. Yet, not all yeast cell walls perform the same and recent research has revealed why.
A closer look at how binding really works
Researchers at Lallemand Animal Nutrition, in collaboration with the Toulouse Biotechnology Institute (INSA Toulouse, France), have developed and published a new in vitro method to measure pathogen binding using flow cytometry. This advanced approach quantifies bacteria-yeast interactions at the single-cell level, bringing new precision and reliability to functionality testing.
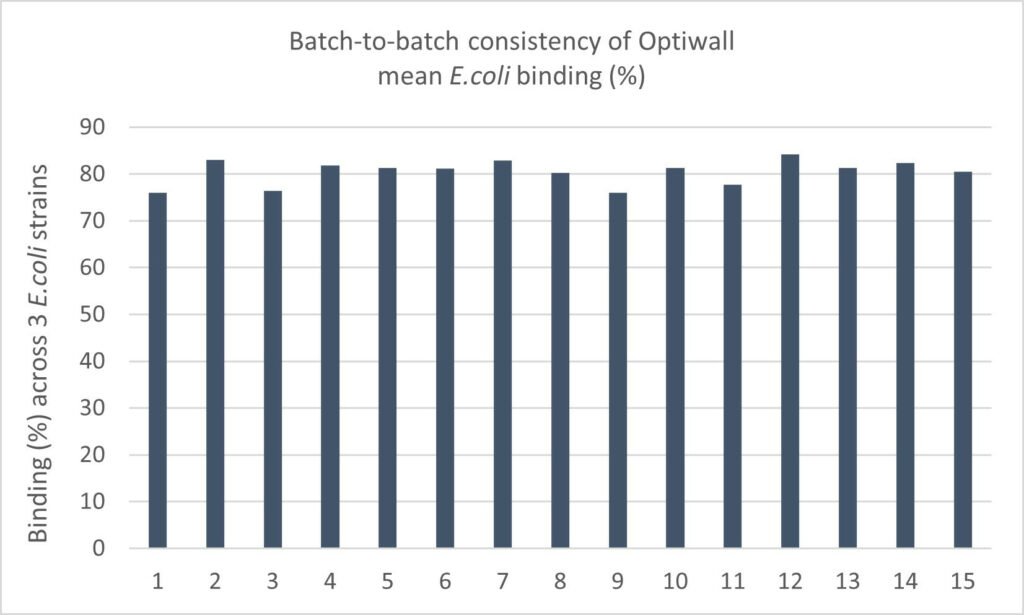
Applied to Optiwall (Lallemand Animal Nutrition), a yeast cell wall product with distinct structural features, this method confirmed powerful and consistent binding across multiple E. coli and Salmonella strains. Testing over 15 batches on different E. coli strains (Figure 1) demonstrated strong batch-to-batch consistency and broad-spectrum versatility, providing robust validation of Optiwall’s performance and reliability. On average, Optiwall achieved around 80% binding across 3 E. coli strains and 96% with Salmonella, highlighting its strong and consistent pathogen-binding capacity.
Figure 1 – Across 15 production batches, Optiwall demonstrated an average pathogen-binding rate of 80.4 ± 2.65% (mean ± SD, n = 15), confirming strong consistency and process reliability (Lallemand, internal data, 2025).
Structure matters more than numbers
The results bring a clear message: efficacy cannot be predicted by composition alone. Even products with comparable or higher MOS or β-glucan levels may differ widely in functionality. What truly makes the difference is how the yeast cell wall is built: its strain origin, structural integrity, and the length and accessibility of its MOS chains.
Optiwall’s distinctive strength lies in its thick, cohesive cell wall and long-chain MOS, which offer effective binding sites for bacterial fimbriae, the tiny “hooks” pathogens use to anchor themselves in the gut. This unique structure, preserved through a controlled and gentle production process, turns Optiwall into a reliable tool to help maintain gut health by naturally by preventing the adhesion of undesirable bacteria that would normally occur on the gut epithelium.
Figure 2a and 2b – Scanning electron microscopy (SEM) images showing Optiwall yeast cell walls with bound Escherichia coli (E. coli).

From specification to functionality
With this breakthrough method, Lallemand brings new insight to yeast cell wall science. It shifts the focus from specifications to functionality, emphasising measurable biological outcomes. Optiwall stands for a new generation of yeast solutions, scientifically substantiated, structurally optimised, and designed to deliver consistent efficacy support gut health, animal welfare, and performance.